VALIDATION OF VERTICAL AUTOCLAVE EQUIPMENT ID – ------
PROTOCOL CONTENTS
|
S. No. |
Description |
Page No. |
|
1.0 |
Protocol approval |
3 |
|
2.0 |
Overview |
4 |
|
2.1 |
Objective |
4 |
|
2.2 |
Purpose |
4 |
|
2.3 |
Scope |
4 |
|
3.0 |
Responsibility
and Validation team |
4 |
|
4.0 |
Training |
5 |
|
5.0 |
Equipment/System
description |
5 |
|
6.0 |
Validation Procedures |
6 |
|
6.1 |
Flow charts |
6 |
|
6.2 |
Validation
Matrix |
7 |
|
6.3 |
Pre –
validation test requirements |
7 |
|
6.4 |
Verification
of Calibration of gauges |
8 |
|
6.5 |
Empty Chamber
Heat Distribution Studies |
8 |
|
6.6 |
Loaded Chamber
Heat Penetration Studies |
10 |
|
6.7 |
Developmental Approach |
15 |
|
7.0 |
Discrepancy and corrective action report |
14 |
|
8.0 |
References |
14 |
|
9.0 |
Summary |
14 |
|
10.0 |
Conclusions |
15 |
|
11.0 |
Attachment |
15 |
|
12.0 |
Revalidation
Criteria |
15 |
|
13.0 |
Revision History |
16 |
|
Signing
of this approval page of protocol indicates agreement with the qualification
approach described in this document. If any modification in the equipment
qualification approach becomes necessary, a revision through change control
shall be prepared, checked and approved. This protocol cannot be executed
unless approved by following personnel. |
|||
|
Department |
Name |
Designation
|
Signature /Date |
Prepared by
|
|||
|
Microbiology |
|
|
|
|
Quality
Assurance |
|
|
|
Reviewed by
|
|||
|
HOD-Engineering |
|
|
|
|
HOD-Production |
|
|
|
|
HOD-Microbiology |
|
|
|
|
Quality
Assurance |
|
|
|
Authorised by
|
|||
|
HOD –
QA |
|
|
|
2.0
Overview
The Vertical autoclave is used to
decontaminate the media containing petri-plates, bottles, sterility canisters
and the media test tubes before media discard.
This
protocol is designed to establish sufficient data, to assure that the Vertical Autoclave
(QCVAC-01) is suitable for decontaminating the defined load patterns when
operated in accordance with the established standard operating procedure as per
SOP.
2.2
Purpose
The
purpose of this protocol is to help the operating personnel to plan the
validation activities so as to demonstrate that the vertical autoclave operates
consistently to decontaminate the media and there are no remaining microbes
alive in the media (which is to be discarded).
2.3
Scope
This Performance Qualification
Protocol is applicable for the Laboratory Vertical Autoclave (QCVAC-01)
installed in Microbiology section at ----------
3.0
Responsibilities
and validation team
Responsibilities: The
group comprising of representatives from each of the following departments and
they shall be responsible for the overall compliance with this protocol.
Department
|
Responsibility
|
|
Engineering & Utility
|
Arranging for execution and recording of the
following activities
|
|
|
·
Calibration of gauges and
instruments ·
Periodic calibration of data
logger during validation ·
Review of protocol and report ·
Coordination with validation
activities and maintaining records. |
|
|
|
Microbiology |
· Execution and collection of data. · Participate and provide the necessary support
for the validation. · Carryout
the analysis of the Biological indicators. · Review of Protocol by department HOD/ In
charge |
|
Out side contractor
|
· Participate
and Provide necessary support for the validation activity
(as applicable). |
|
Quality
Assurance
|
· Verifying
the compliance of existing procedures to the outcome of validation. · Preparation,
Review of protocol and report. · Provide
training to concern personnel on the execution of validation activities. · HOD
is responsible for final approval of protocol and report. |
|
4.0
Training
The validation team member shall be
trained on the protocol execution of validation activity and report compilation.
The
training record shall be attached.( format No.QAGN006/F08)
5.0
Equipment Description
5.1
Equipment Detail:
|
Equipment
Name |
Vertical Autoclave |
|
Make |
Equitron |
|
Serial
Number |
7431FA.14B |
|
Equipment
ID |
QCVAC-01 |
|
Location |
Microbiology Lab |
5.1.1
Vertical Autoclave is used for the
Decontamination of used materials in microbiology area.
5.1.2
Vertical Autoclave is Vertical
cylindrical vessel, it can be operated in auto mode and having the jacketed
chamber with one round lid. As per defined load pattern, objects, which are to
be decontaminated, should be loaded vertically.
5.2
Working
Principle
5.2.1
The steam is prepared by pouring
purified water into the autoclave upto the Heater cover X stand inside. This
prepares steam at 121ºC.
5.2.2
This cycle is controlled by a Microprocessor
which controls the heating coil when the temperature goes above set temperature
range.
5.2.3
The Vertical autoclave has one
temperature sensor which controls the functioning.
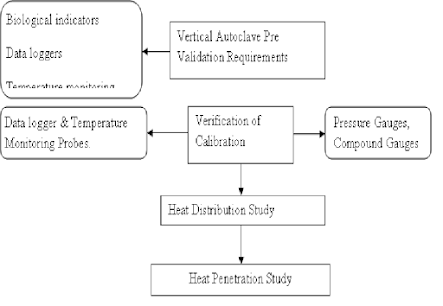
6.0
Validation Test Procedures
6.1
Flow
Chart
6.2 Validation
Matrix
6.2.1 Frequency of periodic monitoring is
1Year ± 30Days
6.2.2 The following test matrix is
prepared for the Periodic Monitoring and
Periodic Monitoring of Autoclave cum
Bung Processor.
Sr. No. |
Test Description |
Periodic
Monitoring |
|
|
Performance
Qualification |
Revalidation |
||
|
No. of Cycle |
No. of Cycle |
||
|
|
Developmental Run* |
1 |
1 |
|
|
Empty chamber Heat Distribution Study |
3 |
1 |
|
|
Loaded Chamber
Heat Penetration Study |
||
|
|
a)
Petriplate and
Bottle Load |
3 |
1 |
|
|
b)
Test Tube and
Bottle Load |
3 |
1 |
* Incase of new load introduction one
developmental run shall be taken.
Note: - Any Change in Load
pattern/process/items three consecutive run are required successful validation.
6.3
Pre-validation
test requirements:
6.3.1
Following instruments shall be
required for the validation of Vertical autoclave used in the Microbiology Area.
·
Calibrated data loggers
·
Calibrated Temperature mapping
probes.
·
Geobacillus
stearothermophillus i.e. Biological Indicator Strips/Ampoules
6.3.2
The Decontamination autoclave shall
be qualified after verification of the documented evidence (obtained as per the
methods outlined in this protocol) assuring that the equipment is meeting the
desired performance attributes consistently.
6.3.3
The following pre-validation tests
shall be performed:
|
S. No. |
Description of
Activity |
Frequency |
|
1. |
Verification of calibration status of various
Instruments associated with Decontamination Autoclave. |
Before
start of the Validation activity. |
|
2. |
Verification of calibration status of data Logger
& sensors |
Before
start of the Validation activity. |
6.4
Verification
of calibration of gauges and Instruments:
6.4.1
It is necessary to verify and ensure
the Calibration status of Gauges and instruments, before carrying out
validation tests.
|
Instrument |
Range |
I.D.
No. |
Location |
Calibrated Date |
Next Due Date |
|
Pressure
Gauge |
2.1 Kg/cm²; 0 – 30 psi |
QCVPG-01 |
Lid of Autoclave |
|
|
|
Temperature
Controller |
0 – 150 ºC |
QCVTC-01 |
With HMI |
|
|
|
Temperature Sensor |
0 – 150 ºC |
QCVTS-01 |
With HMI |
|
|
|
Data
logger |
|
|
|
|
|
|
Data
logger & Sensors in loop |
|
|
|
Pre- Calibration |
Post- Calibration |
|
|
|
6.4.2
Testing and measuring instruments
related to equipment shall be verified, recorded in the report for the
verification of their calibration status.
6.5
Empty Chamber Heat Distribution
Studies
6.5.1.1
The
Autoclave is capable of attaining a temperature of 121.1°C during the decontamination
hold period of 30 minutes.
6.5.1.2
Analyze
the temperature profile and identify the cold spot.
6.5.2.1
Set
the temperature of 122°C for 30 minutes decontamination cycle.
6.5.2.2
Prepare
at least 06 Nos. calibrated temperature mapping probe with location and channel
tag.
6.5.2.3
Pass
06 No. temperature mapping probe into chamber through the validation port of
the Autoclave. Seal the port with
silicone sealant so that steam leakage does not take place. Suspend the probes in the chamber in
different position as per the drawing no. 1 and ensure that probes do not touch
any metallic surface.
6.5.2.4
Connect
the probes to a suitable data logger, which can scan and print the actual
temperature observed at different locations with respect to time.
6.5.2.5
Operate
the Vertical Autoclave as per SOP No. QC-SP-340.
6.5.2.6
The
decontamination hold time for an empty chamber shall be 30 minutes.
6.5.2.7
Start
the data logger to record actual temperatures within the decontamination
chamber with respect to time at a scan time of NMT 15 sec interval.
6.5.2.8
After
completion of cycle connect the data logger to the computer system and download the
data and calculate Fo value of each temperature mapping location.
6.5.2.9
Compile
the data generated during the qualification test for a complete evaluation of the
system.
6.5.2.10 Prepare summary and conclusion of the performance qualification test, which will be finally approved by Head - Quality Assurance.
6.5.3
Acceptance Criteria
6.5.3.1
The
temperature at each temperature mapping probe should be within the range of 121.1°C
to 124°C during the decontamination hold period of 30 min.
6.5.3.2
The
minimum Fo should be 30 min (based on process lethality during the decontamination
cycle).
6.5.3.3
The
pressure during decontamination hold period should be 1.1 - 1.2 Kg/cm².Observation
and Result
6.5.3.4
d
6.6
Loaded Chamber Heat Penetration
Studies
6.6.1
Objective
6.6.1.1
The
Autoclave is capable of attaining a temperature of 121.1°C within the defined
loads during the decontamination hold period of 30 minutes.
6.6.1.2
Find
out the cold spot if any.
|
Sr. No. |
Load Description |
Material |
Quantity (Maximum Load) |
|
1 |
Petri
plate and bottle Load (Media
Load- 1) |
Petriplate |
Full Autoclavable
bag (80 nos.) |
|
Bottles |
05
Nos. |
||
|
Spreader |
10
Nos. |
||
|
10
ml Test tubes (Small) |
1
Stand (40 nos) |
||
|
Tips |
1
Box (50 tips) |
||
|
Sterility
Canisters |
10
Box |
||
|
2 |
Test
Tubes and bottle load (Media
Load- 2) |
100
ml Test Tubes (Large) |
2
Full Stand (20 nos. Each) |
|
Bottles |
05
Nos. |
||
|
Spreader |
10
Nos. |
||
|
10
ml Test Tubes (Small) |
1
Stand (40 nos) |
||
|
Tips |
1
box (50 tips each) |
||
|
Sterility
Canisters |
10 |
6.6.3
Procedure
6.6.3.1
Set
the temperature of 122°C for 30 minutes decontamination cycle.
6.6.3.2
Prepare
at least 06 Nos. calibrated temperature mapping probe with location tag.
6.6.3.3
Load
the chamber Pass 06 No. temperature mapping probe into chamber through the
validation port of the Autoclave. Seal
the port with silicone sealant so that steam leakage does not take place. Suspend the probes in the chamber in
different position as per the photographic representation and ensure that
probes do not touch any metallic surface.
6.6.3.4
Connect
the probes to a suitable data logger, which can scan and print the actual
temperature with respect to time.
6.6.3.5
Attach
the Biological Indicators to each probe as per the drawing no. 2
6.6.3.6
Operate
the Vertical Autoclave as per SOP No. QC-SP-340.
6.6.3.7
The
decontamination hold time for loaded chamber heat penetration shall be 30
minutes.
6.6.3.8
Start
the data logger to record actual temperatures within the decontamination
chamber with respect to time at a scan time of NMT 15 sec interval.
6.6.3.9
After
completion of cycle connect the data logger to computer system and download the
data and calculate Fo value of each temperature mapping location.
6.6.3.10
Compile
the data generated during the qualification test for complete evaluation of the
system.
6.6.3.11
Prepare
summary and conclusion of the performance qualification test, which will be
finally approved by Head - Quality Assurance.
6.6.4.1
The
temperature at each temperature mapping probe inside the load should be within
the range of 121.1°C to 124°C during the decontamination hold period of 30 min.
6.6.4.2
The
minimum Fo should be 30 min (based on process lethality during the decontamination
cycle).
6.6.4.3
No
growth should observe in exposed biological indicators after incubation at
specified temperature and duration. The positive control should show growth
after incubation.
6.6.4.4
The
pressure during decontamination hold period should be between 1.1 - 1.2
Kg/cm².
6.6.4.5
Record
the observations and results in the enclosed Annexure - II.
6.6.5
Photographic Representation Location of temperature sensor and biological
indicator
6.6.5.1
Petriplate and bottle load
External
Sensor
Biological Indicator Strip
6.6.6
Justification
for the probe location
|
Sr.No |
Load Description |
Probe No |
Probe Location |
Justification |
|
01 |
Empty
Cycle |
01 |
Lower frame left side |
Inside Chamber to monitor
temperature profile at Lower frame left side |
|
02 |
Lower frame right side |
Inside Chamber to monitor
temperature profile at Lower frame right side |
||
|
03 |
Middle of chamber |
Inside Chamber to monitor
temperature profile at Middle of chamber |
||
|
04 |
Middle of chamber |
Inside
Chamber to monitor temperature profile at Middle of chamber |
||
|
05 |
Upper frame in the middle |
Inside
Chamber to monitor temperature profile at Upper frame in the middle |
||
|
06 |
Inside chamber with external probe |
Inside Chamber to monitor
temperature profile at Inside chamber with
external probe |
||
|
02 |
Petriplate
and bottle load (Media
Load- 1) |
01 |
Lower frame inside the
Petriplates |
Inside
the load to monitor temperature profile at Lower frame inside the Petriplates |
|
02 |
Upper frame inside the Bottle |
Inside
the load to monitor temperature profile at Upper frame inside the Bottle |
||
|
03 |
Upper frame near the Spreader |
Inside
the load to monitor temperature profile at Upper frame near the Spreader |
||
|
04 |
Upper frame inside Test tube |
Inside
the load to monitor temperature profile at Upper frame near the Spreader |
||
|
05 |
Upper frame inside Sterility canisters |
Inside the load to monitor
temperature profile at Upper frame inside Sterility canisters |
||
|
06 |
Inside Chamber with external
probe |
Inside
Chamber With RTDs to monitor temperature profile at Inside Chamber with
external probe |
||
|
03 |
Test
tubes and bottle load (Media
Load- 2) |
01 |
Upper frame inside the Large Test tubes |
Inside the load to monitor
temperature profile at Upper frame inside the Large Test tubes |
|
02 |
Upper frame inside the bottle |
Inside
the load to monitor temperature profile at Upper frame inside the bottle |
||
|
03 |
Upper frame inside Spreader |
Inside
the load to monitor temperature profile at Upper frame inside Spreader |
||
|
04 |
Lower frame inside the small test
tubes |
Inside
the load to monitor temperature profile at Lower frame inside the small test
tubes |
||
|
05 |
Lower frame Inside Sterility
Canister |
Inside
the load to monitor temperature profile at Lower frame Inside Sterility
Canister |
||
|
06 |
Inside Chamber with external
probe |
Inside
Chamber With RTDs to monitor temperature profile at Inside Chamber with
external probe |
6.7
Developmental Approach Study
6.7.1
Developmental studies shall be
carried out for assessing the parameters at different set parameters and time
intervals to achieve the predefined objective/approach.
6.7.2
The developmental study to be
continued up to achieving of the defined goal,
6.7.3
After achieving the defined goal the
cycle shall be repeated 3 times to validate the defined parameters or changes
in load or process.
6.7.4
The complete details of the
developmental study shall be recorded in Annexure - III
7.0
Discrepancy and corrective Action Report
Any
discrepancies observed during the validation of the equipment shall be
justified in the summary report. Corrective actions of the same shall also be
included. For resolving the discrepancy an action plan shall be developed.
8.0
References
SOP on operation,
Calibration, Cleaning and Maintenance of Vertical autoclave.
9.0
Summary
The validation
report shall include a summary of validation activities describing the
following in brief
9.1
Protocol reference
9.2
Dates of validation activities
9.3
Confirmation of calibration and
preventive maintenance status of instruments, probes and Vertical Autoclave.
9.4
Heat Distribution Study
9.5
Heat Penetration Study
9.6
Brief on result obtained
9.7
Log reduction of Biological
Indicators
10.0
Conclusion
The validation report
shall include statement for conclusion of the validation activity based on
result and observation from the validation activity.
11.0
Attachment
The following
are the attachments which shall be used for collecting and evaluating the raw
data.
|
Sr. No |
Test data sheet |
Title |
Pages |
|
1 |
Annexure – I |
Empty
Chamber Heat Distribution |
5 Pages |
|
2 |
Annexure – II |
Loaded
Chamber Heat Penetration |
6 Pages |
|
3 |
Annexure – III |
Developmental
Approach Study |
5 Pages |
12.0
Revalidation Criteria
Revalidation
criteria is as follows
·
If any major changes or modification in
the system.
·
If any Major changes have been done in
the respective room or module, which is affecting the environmental condition.
·
If there is a contamination problem.
·
At the time of relocation or
Re-qualification.
·
At an yearly frequency as per schedule.
13.0
Revision History
|
Document No. |
Effective Date |
Nature of Change/ Reason for
Revision |
|
PRT/VL/VAC/01/00 |
20-07-2013 |
1
New Protocol |
|
PRT/VL/VAC/01/01 |
22-07-2014 |
1
Removal of typographical error 2
Introduction of validation Matrix 3
Addition of the Developmental
Approach Study 4
Explanation of Procedure i.e. a)
Changes of the decontamination set
temperature from 1220C to
121.1 and b)
Replacement of the word decontamination
with decontamination. |






No comments:
Post a Comment
Pharmaceutical guideline only